|
Singly ionized sulfur monoxide (SO+) was detected by
Turner in 1992 in the "shocked molecular clump"
IC 443G.
The observations were made with the
NRAO 12m radio telescope.
Turner suggested that SO+ was evidence for shock chemistry, that it was formed via a dissociative
shock. However, van Dishoeck et al. countered
that SO+ could be formed without a shock, if S+ is present in pre-shocked gas.
|
|
|
|
SO+ has also been detected toward the quasar
PKS 1830-211, as reported by
Muller et al in 2011.
See Holger Müller's
entry
on extragalactic SO+ for more details about the detection.
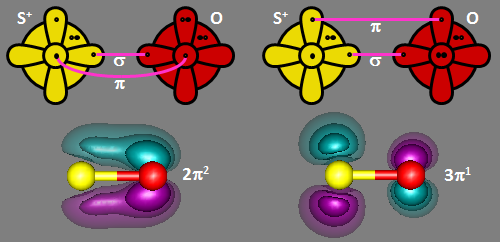
When SO is ionized, the electron is removed from the sulfur 3p2 orbital, leaving
a phosphorus-like 3s2 3px 3py 3pz configuration. That means that when
O bonds to it, there is one σ bond and one π bond, with one singly occupied orbital leftover on sulfur.
There are two ways to do this, as shown below in the bonding diagrams below. SO+ has a
2Π ground state, which gives it distictively doubled lines. The doubly and singly occupied valence
π orbitals are also shown.
|